Written by Jessica Nimon
International Space Station Program Science Office
NASA’s Johnson Space Center
 Houston, TX – As the saying goes, sticks and stones may break your bones—especially if you have a weak skeleton. This is not only a concern for the elderly who can suffer from osteoporosis. Inactivity from injury, illness, or malnutrition from anorexia or dietary challenges also can lead to bone breakdown in otherwise healthy people.
Houston, TX – As the saying goes, sticks and stones may break your bones—especially if you have a weak skeleton. This is not only a concern for the elderly who can suffer from osteoporosis. Inactivity from injury, illness, or malnutrition from anorexia or dietary challenges also can lead to bone breakdown in otherwise healthy people.
Another cause of bone loss is living in microgravity. While most people may never experience life in space, the benefits of studying bone loss aboard the International Space Station has the potential to touch all of our lives here on the ground.
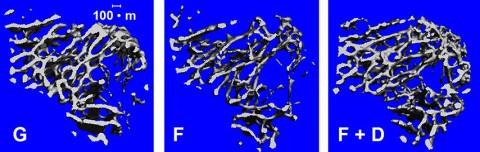
This accelerated aspect of bone loss in spaceflight provides an opportunity for researchers to identify the mechanisms that control bones at a cellular level.
With that goal in mind, researchers looked at rodents flown aboard space shuttle missions to the space station in a series of experiments called the Commercial Biomedical Testing Module (CBTM) investigations.
For the CBTM studies, the rodents lived in a habitat designed for spaceflight—the Animal Enclosure Module (AEM)—developed by NASA’s Ames Research Center in Moffett Field, California. The data from this series of investigations factored into research related to pharmaceuticals for use on Earth to mitigate bone loss, such as Prolia.
“We contributed to preclinical trials of pharmaceuticals to show how well they work to improve bone and/or muscle loss that results from disuse,” said Virginia Ferguson, Ph.D., CBTM principal investigator at BioServe Space Technologies, University of Colorado, Boulder.
Bone remodeling—the natural breakdown and rebuilding of bone—occurs in a balanced fashion in healthy bone so that the rate of rebuilding, known as formation, equals the rate of breakdown and absorption, known as resorption.
This cycle of breakdown and buildup helps us to maintain skeletal strength and repair injuries like fractures so we can continue to enjoy normal mobility. When this natural process is out of balance, our bones and health may suffer.
The CBTM studies were collaborative in nature toward a better understanding of bone health in the science community. CBTM, CBTM 2, and CBTM 3 took place aboard the space shuttles on missions STS-108, STS-118 and STS-135, respectively. Ferguson and her colleagues Louis Stodieck, Ph.D., of Bioserve, and Ted Bateman, Ph.D., of University of North Carolina, Chapel Hill, and their teams then analyzed the data on the ground.
“This [was] an amazing group effort,” said Ferguson. “Many researchers across countless disciplines have benefited from access to the tissues that we collected from mice on STS-108, 118 and 135.”
The 2007 flight studied myostatin, a preclinical therapy for treating muscle loss. All three therapeutics, which were in preclinical development with Amgen during the time of their flights, had positive impacts on maintaining bone strength.
“In all three cases all three drugs had tremendous beneficial effects to the bone, and this is even despite the one for STS-118 being the one for muscle therapeutic—meaning it has both bone and muscle effects,” said Ferguson. “For the myostatin inhibitor, it was surprising. We thought it would have bone effects—it’s a naturally occurring pathway in bone metabolism—but we never anticipated that the bone effects would be as beneficial as they were in this particular model.”
Ferguson elaborated that the therapies studied in the CBTM investigations are favored for pharmaceutical development because they work with the body’s existing methods of breaking down and building up bone. These processes are a necessary part of bone health as we grow and age. The use of a drug such as OPG can turn the natural signaling pathways on or off, encouraging the bone either toward or away from resorption of its cells.
“It’s cool because you can turn back normal bone function after you stop trying to arrest resorption,” said Ferguson. For instance, she continued, “You try to stop bone removal in an astronaut during flight, and when they come back you allow that bone removal to proceed as normal by altering that pathway a little further with another therapeutic. You try to get their bones back to a normal state of remodeling.”
Getting therapies from the lab to the medicine cabinet takes time, as did the progression of these studies—which spanned a decade in orbit as the space station was under construction.
This duration enabled advances in the ways researchers conducted their microgravity investigations, including enhancements to the available tools for analysis.
“Some of our imaging techniques improved dramatically for small animal imaging between the first flight and our third flight,” said Ferguson. “We were able to collect a tremendous amount of data on the third flight through technologies that were not even available on the first one. That was thrilling for us to be able to add to our repertoire that way.”
“We did these investigations on space shuttle flights because that was what was available to us at the time. The [AEM] hardware that they flew the mice in previously had been limited to being approved for use for about the duration of a space shuttle flight,” said Ferguson. The three shuttle flights for the studies lasted 11 to 12 days each. “[Ames engineers have] since made modifications to the hardware that will enable mice to fly for longer periods of time [up to three months]. We are really excited about the potential that these longer periods of time afford us.”
With the space station’s upcoming Rodent Research Facility, the AEM concept was modified to enable the next generation of studies planned for operations in orbit, such as Rodent Research-1 (RR-1). The RR-1 mission will validate the capabilities of the new rodent research hardware and feature a commercial study.
One of CBTM’s investigators, Stodieck, will conduct this research, which is facilitated by CASIS in cooperation with Bioserve and industry partners.
The investigation is planned to launch with the fourth SpaceX commercial resupply mission in 2014 and make use of the various partnerships that enable research aboard the orbiting laboratory. These relationships continue to push the envelope of science in space, seeking answers to propel exploration and benefit people on Earth.
“One thing that can’t be ignored is the tie to the commercialization of space and the integration of these industries,” said Ferguson. “The biotech and pharmaceutical companies are using spaceflight as a medium to study their drugs and do the preclinical work that’s really important for FDA approval.”


